Developed a new companion diagnostic for Keytruda, Merck
May 28, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, WuXi PharmaTech partner Foundation Medicine and Merck (MSD) announced a partnership to develop the Companion Diagnostics (CDx) test for anti-PD-1 tumor therapy Keytruda (pembrolizumab). The collaboration will develop pan-adjuvant concomitant diagnostics around the FDA-approved FoundationOne CDx comprehensive genomic profiling to measure microsatellite instability (MSI), tumor mutation load (TMB) and other potential novel biomarkers.

Foundation Medicine, a molecular information company dedicated to cancer care, seeks treatment by gaining insight into the genomic changes that cause cancer in every patient. The company offers a comprehensive suite of genomic characterization methods to identify molecular changes in cancer patients and match them to relevant targeted therapies, immunotherapies and clinical trials. Foundation Medicine's Molecular Information Platform is designed to advance the daily care of patients and advance cancer molecular medicine by meeting the needs of clinicians, academic researchers and drug developers.
FoundationOne CDx is an in vitro diagnostic device based on Next Generation Gene Sequencing (NGS) for the detection of 324 gene changes, including substitutions, insertions and deletions (indels), copy number changes (CNA), and selection of gene rearrangements. These include genomic signatures such as MSI and TMB for isolating DNA from tumor tissue specimens. FoundationOne CDx has been approved by the FDA for patients with certain types of non-small cell lung cancer, melanoma, colorectal cancer, ovarian cancer or breast cancer. In addition, FoundationOne CDx is designed to provide tumor mutation analysis for qualified health care professionals to guide patients with malignant solid tumors based on oncology guidelines.
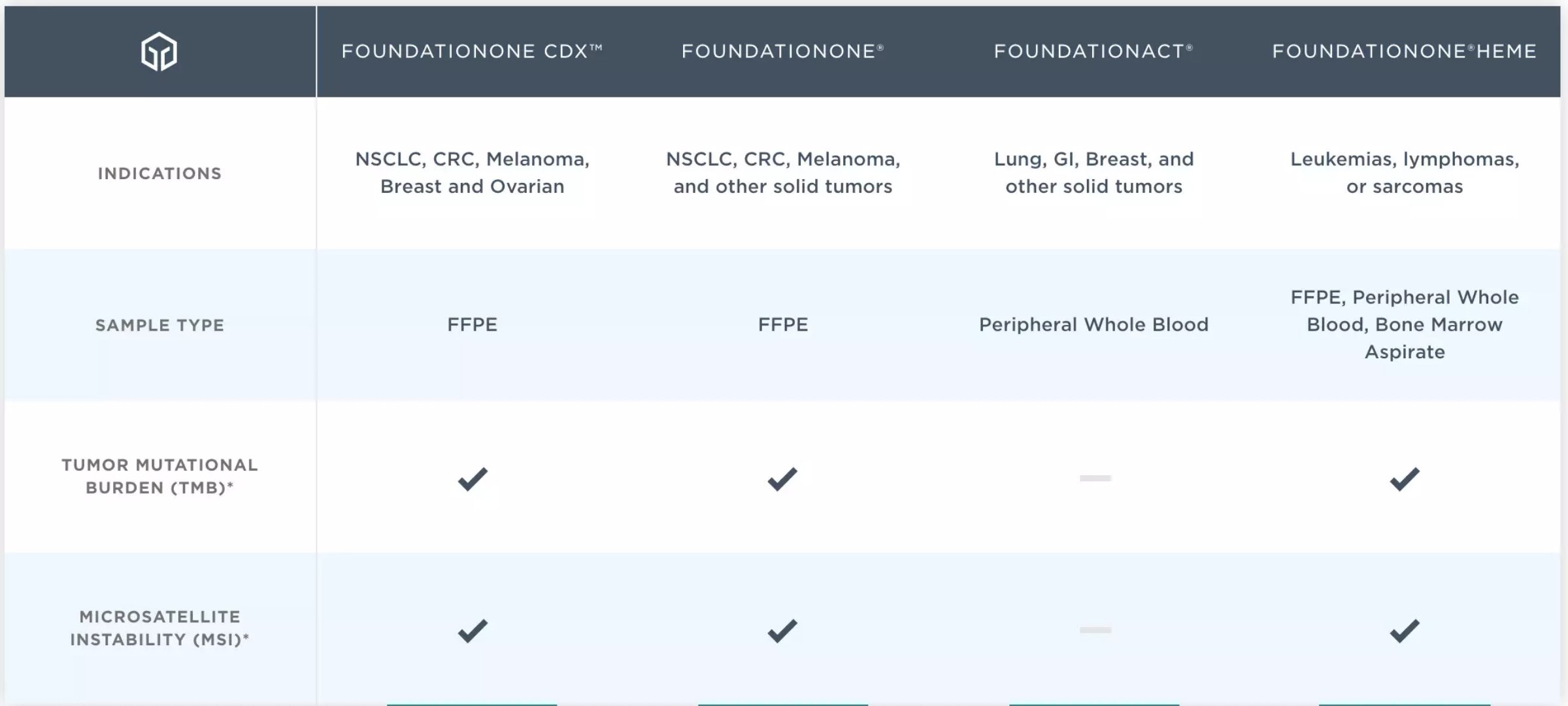
â–²Foundation Medicine genomic diagnosis products (Source: Foundation Medicine official website)
“By cooperating, Merck and Foundation Medicine can continue to drive innovation in the field of immuno-oncology and individualized cancer treatment, making positive changes in the lives of cancer patients,†said Melanie Nallicheri, chief medical officer and head of biomedical division at Foundation Medicine. "The addition of MSI and TMB companion diagnostics to the FoundationOne CDx reaffirms the effectiveness and clinical utility of these key tumor immunobiomarkers. By using a test to simplify the diagnosis, the physician is provided with the necessary information. According to each patient Genomic and biomarker status exclusion and increased potential treatment options can help patients accelerate access to personalized care."
Dr. Eric Rubin, Senior Vice President of Cancer Clinical Development at Merck Research Laboratories, said: "The rapid development of cancer biology and cancer immunology continues to improve our understanding, allowing us to rationally deploy drugs for the most effective patient. Mitigation. We look forward to working with Foundation Medicine on this complementary diagnostic strategy."
We look forward to this collaboration to accelerate the development of effective cancer detection methods and enable patients to rehabilitate with personalized medicine as soon as possible.
Reference materials:
[1] Foundation Medicine Establishes Immuno-OncologyCompanion Diagnostics Collaboration with Merck
[2] Foundation Medicine official website
Food Additives,Natural Food Additives,Food Additive Saccharin,Popular Food Additives
SHANDONG BAISHENG BIOTECHNOLOGY CO., LTD , https://www.baishengbioproducts.com