Migraine new drug phase 3 clinical arrival end, will be submitted for marketing application
March 27, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Today, Biohaven Pharmaceutical has announced positive results for its two Phase 3 clinical trials. The oral new drug rimegepant brought by this company is superior to placebo in the treatment of migraine, reaching a common primary end point.

Migraine is a neurological disease that affects a wide range. Migraine is estimated to be the third most common epidemic in the world, with 36 million patients in the United States alone. According to another survey, migraine is the seventh leading cause of global disability, with more than 90% of patients failing to work properly during a migraine attack. Due to its universality and severity, migraine has received the attention of many new drug developers. However, many patients will still have a headache recurrence within 24 hours after the current treatment. They also urgently need new drugs to control their condition.
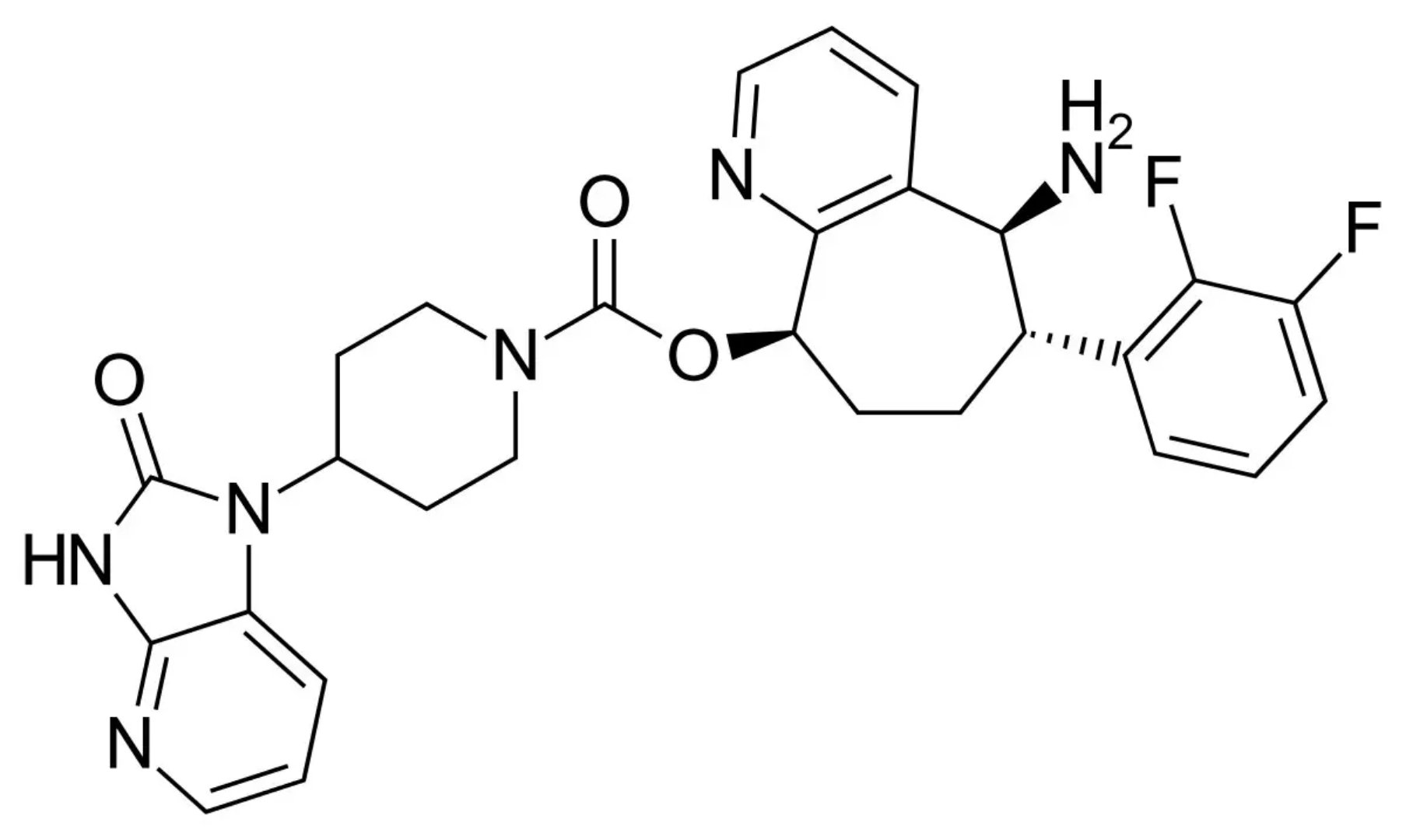
â–²Rimegepant's molecular structure (Source: By Vaccinationist (Own work, based on PubChem) [Public domain or Public domain], via Wikimedia Commons)
Rimegepant is an oral CGRP receptor antagonist that is expected to treat migraine through this recently popular pathway. Studies have shown that the patient's headache symptoms have improved within 8 hours of treatment. Two hours after administration, the proportion of patients who no longer had headaches in the two clinical trials was 19.2% and 19.6%, respectively, compared with 14.2% (p < 0.03) and 12.0% (p < 0.001) in the placebo control group. After 2 hours, rimegepant also showed a gratifying effect. Compared with placebo, patients who received rimegepant increased their rate of no more headaches by 5%-19% (first trial) and 7%-22% (second trial).
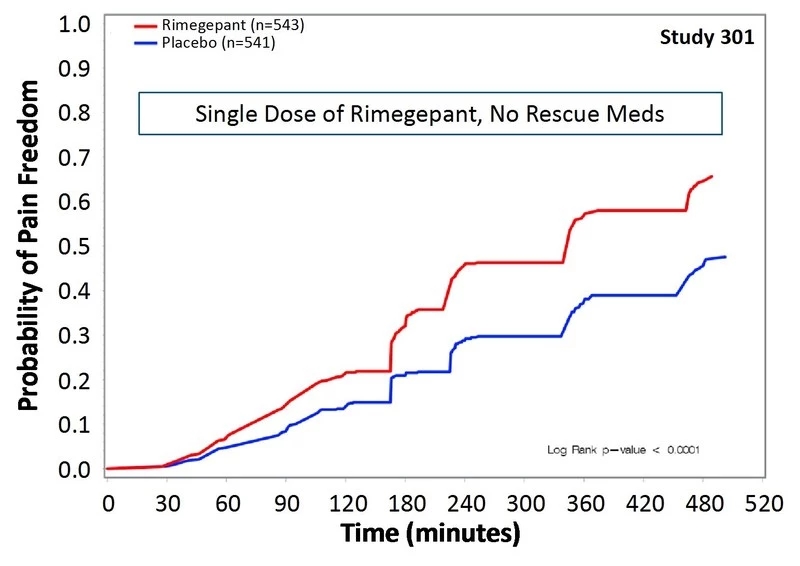
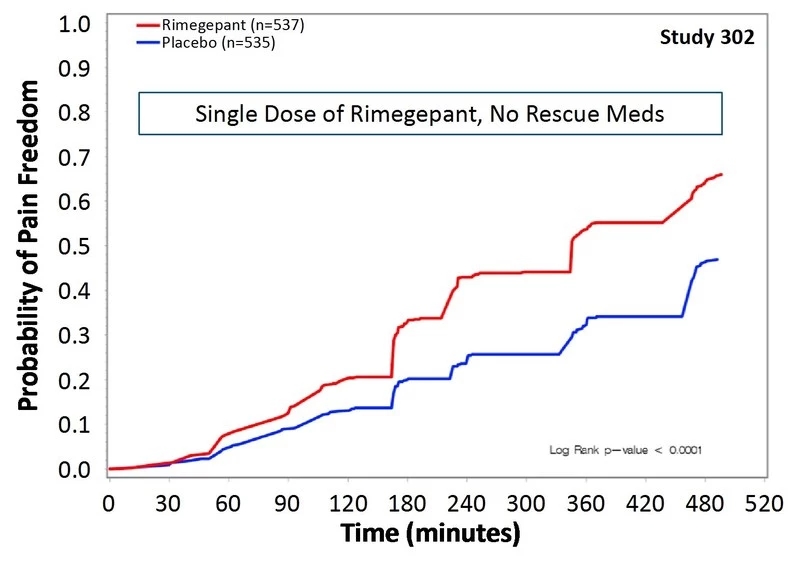
â–² Two clinical trials show that this new drug can work quickly (Source: Biohaven official website)
On other indicators, patients who received rimegepant also showed significant improvement. This new drug also demonstrates fast-acting, well-tolerated features.
“The top-line results of these two key clinical trials indicate that a single oral dose of rimegepant has the potential to be a fast and effective treatment for migraine. It can solve headaches and other disturbing symptoms without repeated medication. Combining positive efficacy and safety In view of this, we believe that rimegepant is expected to bring significant improvements beyond the existing treatment options. We thank patients, researchers, and Biohaven employees for their efforts to bring this candidate to this important milestone.†Biohaven Dr. Vlad Coric, CEO, said.
“Doctors have been waiting for migraine innovations for a long time,†said Dr. Richard B. Lipton, a professor of neurology at Albert Einstein Medical School. “The results of these two studies are very exciting. Rimegepant has reached the regulatory end point and has potential Bring important clinical benefits to patients."
According to the plan, Biohaven will submit the listing application in 2019. We expect this new drug to bring the gospel to the majority of migraine patients!
Reference materials:
[1] Biohaven Announces Successful Achievement of Both Co-Primary Regulatory Endpoints in Two Pivotal Phase 3 Trials of Rimegepant an Oral CGRP Receptor Antagonist for the Acute Treatment of Migraine
[2] Biohaven Official Website
Amino acids are biologically important organic compounds composed of amine and carboxylic acid functional groups, along with a side-chain specific to each amino acid. With biological significance, amino acids are important in nutrition and are commonly used in nutritional supplements, fertilizers, and food technology. Industrial uses include the production of drugs, biodegradable plastics, and chiral catalysts.
Natural Amino Acids,Amino Acids Powder,Amino Acids Particles,Amino Acids Tablets
SINOCHEM PHARMACEUTICAL CO., LTD , https://www.sinochemnutrition.com