
Article introduction:
Recently, Zhou Xuedong and Yuan Quan, from the West China Hospital of Sichuan University, and the Lin Shuibin team of the First Affiliated Hospital of Sun Yat-sen University jointly developed a new study on Mettl3-mediated methylation of m6A RNA to regulate the fate of bone marrow mesenchymal stem cells and osteoporosis. mechanism. The study, titled Mettl3-mediated m6A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis, was published online November 14 in Nature Communications (IF: 12.353).

Research ideas
The research team first constructed a conditional knockout of MSCs and knocked into Mettl3 model mice using CRISPR-Cas9 technology. Micro-CT and staining showed knockdown resulted in low bone mass and high bone marrow fat, and m6A overall methylation in MSCs. At the same time, Mettl3 overexpression prevented osteoporosis caused by estrogen deficiency compared with model rats who underwent oophorectomy; Mettl3 deletion was found by m6A MeRIP-seq and RNA-seq techniques. The global down-regulation of PTH-regulated gene expression in MSCs suggests that the PTH/Pth1r signal axis may be a pathway in which Mettl3-mediated methylation of m6A affects the fate of MSCs. The PTH intermittent injection experiment also confirmed the conclusion that m6A affects the osteogenesis and fat differentiation of MSCs by regulating the PTH/Pth1r signal axis. In addition, overexpression of Pth1r can largely reverse the oblique differentiation of MSCs into adipocytes and improve osteogenesis, further confirming that PTH/Pth1r signaling pathway is a new mechanism of mettl3-mediated m6A modification to control MSCs lineage distribution.
research content
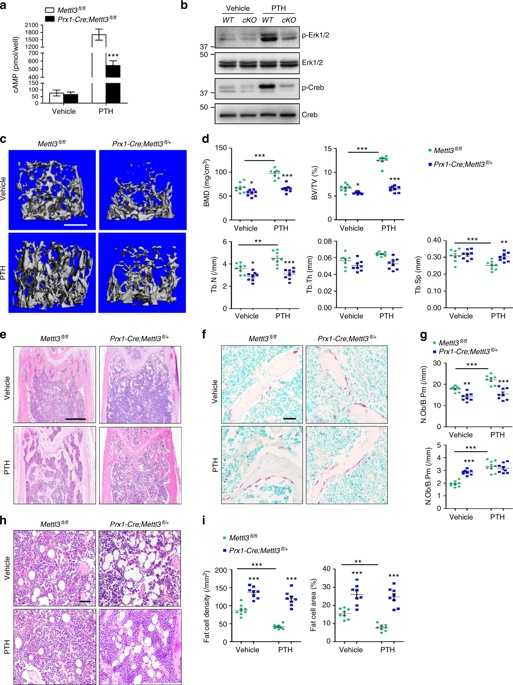
1. Mettl3 knockout in MSCs can lead to low bone mass, high bone marrow fat <br> Using CRISPR-Cas9 technology to construct MSCs conditionally knockout Mettl3 model mice, femoral paraffin section immunostaining and WB validate Mettl3 Knockout is successful. The results showed that Mettl3 knockout mice had significant bone mass loss and accumulation of bone adipose tissue compared with the control group, and their skeletal characteristics were similar to those of osteoporosis. At the same time, LC-MS/MS detection showed that the overall level of m6A methylation in MSCs decreased.
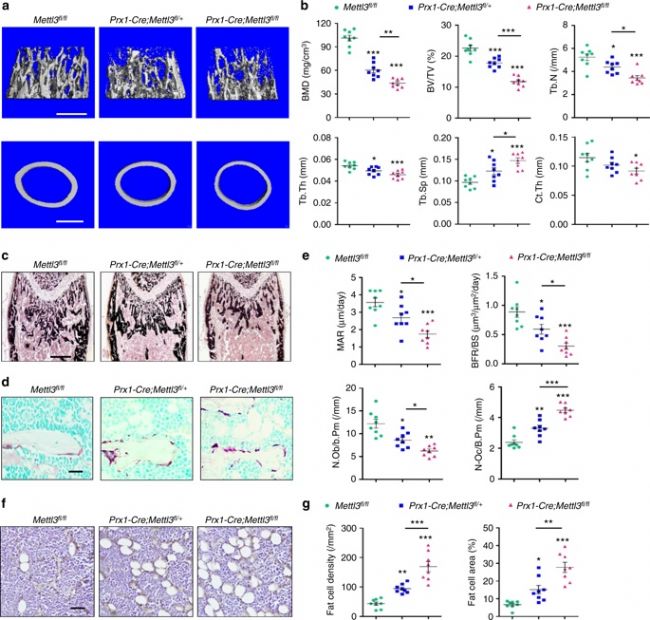
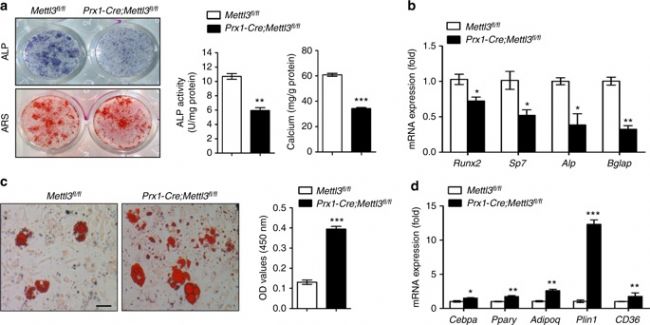
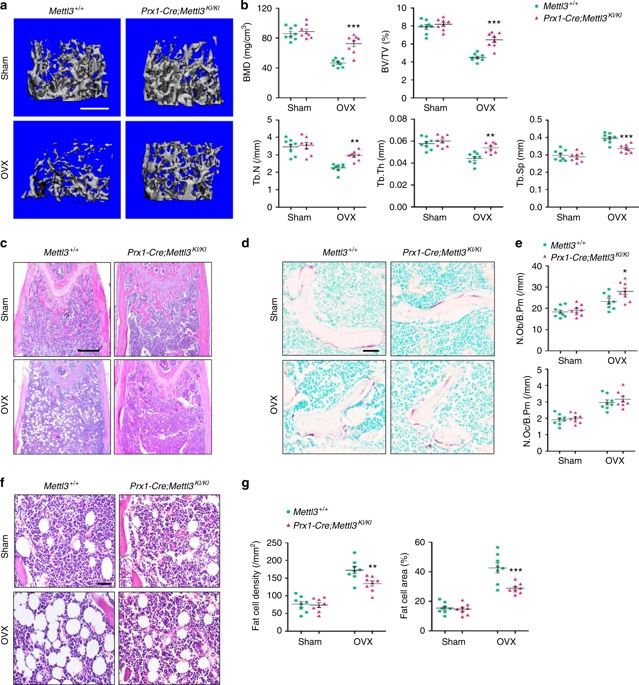
3. Mettl3 overexpression prevents osteoporosis caused by estrogen deficiency <br> To confirm whether Mettl3 overexpression can enhance bone health or prevent bone disease, conditionally knocked Mettl3 into MSCs in MSCs. Overexpression. Osteoporosis experimental model was established by oophorectomy. Compared with the control, Mettl3 overexpression increased the number of osteoblasts in ovariectomized mice, but did not affect osteoclast activity. H&E staining and histomorphometric analysis showed that the ovariectomized mice had excessive accumulation of bone marrow adipocytes, whereas the ovariectomized Mettl3 knock-in mice had a smaller increase in bone marrow adipocytes.
4. m6A regulates the translation of parathyroid hormone receptor-1 (Pth1r) <br> The m6A MeRIP-seq technique is used to analyze the specific situation of m6A in MSCs. The results show that the m6A peak is mainly located in the CDS and 3'UTR regions, and mainly Located near the stop codon. Pth1r was also found to be a key regulator of lineage distribution in MSCs and osteoblast precursors, with high enrichment and specific m6A peaks near its translation stop codon. RNA-seq revealed a global down-regulation of parathyroid hormone (PTH)-regulated gene expression in Mettl3-deficient MSCs, suggesting that the PTH/Pth1r signal axis may be a pathway in which Mettl3-mediated m6A methylation affects the fate of MSCs.
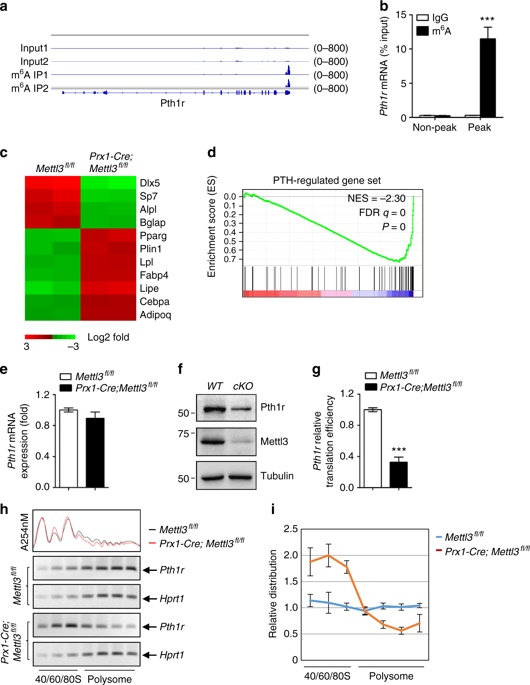
5. m6A is essential for PTH function
Mettl3 knockout mice showed no significant enhancement of bone remodeling after PTH treatment, demonstrating that knockdown of Mettl3 attenuates PTH-induced osteogenesis. In addition to being significantly enriched during osteogenesis, PTH also inhibits adipocyte differentiation and promotes bone formation. Intermittent injection of PTH significantly reduced the accumulation of MAT in Mettl3 knockout mice, but it was difficult to reverse the higher bone marrow fat content in Mettl3-deficient mice; Pth1r overexpression improved the intensity of oil red O staining caused by m6A reduction, significantly Increase ALP activity and calcium nodule formation. Overexpression of Pth1r can largely reverse the oblique differentiation of MSCs into adipocytes and improve osteogenesis, further confirming that PTH/Pth1r signaling pathway is a new mechanism of mettl3-mediated m6A modification to control MSCs lineage distribution.
to sum up
Mettl3-mediated m6A RNA methylation modification in eukaryotes has a broad functional impact on stabilizing homeostasis, and any disturbance in m6A levels can lead to dysfunction or disease. Studies here have confirmed that the deletion of Mettl3 in bone marrow mesenchymal stem cells destroys the cell fate of mice, leading to a pathological phenotype of osteoporosis such as reduced bone mass of osteogenic potential and increased bone marrow fat with enhanced osteogenic potential. The effective specific regulation of m6A on bone marrow mesenchymal stem cells was revealed. In addition, the increased function of Mettl3 prevents postmenopausal osteoporosis caused by estrogen deficiency and establishes the indispensable role of Mettl3 in determining the fate of bone marrow MSCs, thus ensuring bone health. The reduction of epigenetic modification of m6A in early bone marrow mesenchymal stem cells inhibits the translation of mammalian Pth1r and blocks its synthesis of PTH during bone accumulation. These functional defects cause the delicate differentiation of MSCs to tilt toward the fat lineage, leading to severe bone loss and excessive MAT accumulation. The experimental data enriched the evidence of m6A regulating stem cell differentiation, revealing the functional link between improperly regulated m6A modification and bone pathological disorder, thus providing a possibility for the development of new strategies for osteoporosis treatment.
Full text link
Https://
Cloud sequence biological RNA methylation product list:
Cloud order related product recommendation:
m6A RNA whole transcriptome methylation sequencing
m5C RNA whole transcriptome methylation sequencing
m1A RNA whole transcriptome methylation sequencing ultra-micro m6A methylation sequencing colorimetric detection of overall methylation level whole transcriptome sequencing
Past review:
How to quickly find LncRNA sequences
Nature|m6A RNA methylation recognition protein YTHDF1 is involved in memory formation
More than 10 points of m6A RNA methylation sequencing article ---- cloud sequence bio-help is not enough, Daniel tells you how to study lncRNA methylation Xiaobai must see! Summary of methods for identification of overall level of RNA methylation
20-point article on RNA methylation, heat and strength coexisting cloud sequence organisms latest m6A "RNA methylation" research summary - non-coding RNA article cloud sequence biology latest "RNA methylation" research summary - Arabidopsis The latest m6A "RNA methylation" research summary - virus articles
Plant Cell: Arabidopsis found a new top-level article on the new m6A RNA demethylation modification enzyme cloud order client, teaching you how to use RIP sequencing to play the molecular mechanism!
2018 Nature Magazine's Breakthrough Research Results--m5C RNA Methylation Cloud Sequence Creature
Nature Breakthrough Research - RNA Methylation Newly Modified m1A Cloud Sequence Creature
Hepatology: m6A RNA methylase METTL3 promotes liver cancer development New mysterious cloud sequence bio-exclusive m5C RNA methylation sequencing
Nature Article Reveals Multiple Functions of RNA Methylation to Fill Epigenetic Modification Blanks: A New Mechanism for RNA Methylation Regulatory Gene Nucleation
Shanghai Yunxu Biological Technology Co., Ltd.
Shanghai Cloud-seq Biotech Co., Ltd.
Address: 3rd Floor, Building 20, No. 518, Zhangzhu Road, Songjiang District, Shanghai 
Telephone Fax Website:
mailbox:
Custom size medical incision protectors are medical devices specially designed to protect surgical incisions from external contaminants and mechanical damage. They are designed to fit snugly over the incision area and provide a barrier against bacteria, fluids, and other harmful substances that could lead to infection.
To create custom-sized medical incision protectors, medical professionals use advanced 3D scanning and printing technology to create an exact mold of the patient's incision area. This mold is then used to create a custom incision protector that perfectly fits the patient's unique anatomy.
These devices are usually made of medical-grade silicone or other biocompatible materials that are safe for use in the human body. They are easy to apply and remove, and can be reused many times.
Custom sized medical incision protectors are commonly used in a variety of surgical procedures, including orthopedic, cardiac, and plastic surgery. They are an important tool in preventing postoperative infection and ensuring optimal healing outcomes for patients.
Wound Protector,Oem Disposable Cut Protector,Oem Wound Retractor,Disposable Incision Retractor
Changzhou Weipu Medical Devices Co., Ltd. , https://www.cnweipumedical.com