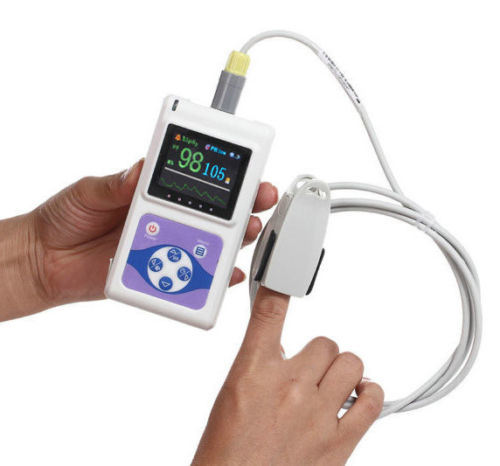


Major Features
Small in volume\light in weight and convenient in carrying
Operation of the product is simple, low power consumption
Operation menu for the function setting
SpO2 value display
Pulse rate value display, bar graph display
Pulse waveform display
Screen brightness can be changed
A pulse rate sound indication
Review function
With measured data overruns limits and low-voltage alarm function, the upper/down alarm range is adjustable
Battery capacity indication)
Low-voltage indication: Low-voltage indicator appears before working abnormally which is due to low-voltage, and with alarm function
With SpO2 value and pulse rate value of storage, the storage data can be uploaded to computers
Real-time data can be transmitted to computers
Connected with an external oximeter probe
Wireless communication function(CMS60DW)
Main performance
Display Mode: 1.8" Color OLED display
Screen Resolution: 160*128
SpO2 Measuring Range: 0%~100%, (the resolution is 1%).
Accuracy: 70%~100%: ± 2%, Below 70% unspecified.
PR Measuring Range: 30bpm~250bpm, (the resolution is 1bpm)
Accuracy: ± 2bpm or ± 2% (select larger)
Measurement Performance in Weak Filling Condition:
SpO2 and pulse rate can be shown correctly when pulse-filling ratio is 0.4%. SpO2 error is ± 4%, pulse rate error is ± 2 bpm or ± 2% (select larger).
Resistance to Surrounding Light: The deviation between the value measured in the condition of man-made light or indoor natural light and that of darkroom is less than ± 1%.
Power Consumption: Less than 100mA
Voltage: DC 3.0V
Power Supply: Alkaline battery(2AA)
Battery Working Hour: Theoretical number is 44 hours.
Safety Type: Interior Battery, BF Type
Accessories
Sell in standard:
A user manual
A data line
A disk (PC software)
An oximeter probe
Sell in addition:
Other oximeter probe(Refer to probe application instruction for details and notice renewal)
Physical Identity
Dimension: 110(L) × 60(W) × 23(H) mm
Weight: About 180g (with Alkaline battery(2AA))
Qualification
CMS60D passed FDA and CE
The Universal Head Immobilizer is a popular first aid product when transfer the patient. Each set is consist of a base, two Head Block and a set head and chin straps. The base fits all our aluminum and plastic backboards. Two hand blocks stop the head moving and the large ear holes on it can monitor the patient. There is the minimal interference with X-ray, MRI, or CT procedures.
High-density foam with water repellent coating makes it not absorb blood or bodily fluids.
Application:
Instruction for Use:
1) Use central top belt
Put base board on Spine Board, insert the top belt through the central hole on spine board
Have belt go through the D loop, reverse and stick on belt hook and loop.
2) Use two belts and the middle four D loop to immobilize base board on spine board.
Immobilize one side hook and loop to D loop
Then turn over the spine board and immobilize the other side to symmetrical D loop.
3) Use two blocks to immobilize patient's head
Firstly immobilize a block on base board's one side.
Put patient's head carefully on the base board.
Put the other Block to the other side of head and get the proper position for head immobilization, stick to hook and loop.
4) Put on two Head Strap
Use one strap and two symmetrical D loop to immobilize forehead.
Use the other strap and left two symmetrical D loop to immobilize the chin.
Universal Head Immobilizer
Head Immobilizer For Ambulance,Head And Neck Immobilizer,Universal Head Immobilization,Head Immobilization Device
Anping Longji Medical Equipment Factory , http://www.azmedicals.com